A microRNA (miRNA) is on the news since Wednesday; many just haven’t been told.
I am referring to AMT-130 by UniQure, the gene therapy slowing the progression of Huntington’s disease [1]. In a nutshell, the miRNA in AMT-130 reduces the level of the protein huntingtin in neurons, which a mutation has turned toxic in Huntington patients. Decreasing the expression of a harmful gene is the most common application for miRNAs in gene therapy, but it is not the only one. And it is not the most ingenious either.
This is a list of 3 medical applications of miRNAs in gene therapies mediated by rAAV, the recombinant adenoassociated virus that UniQure uses to deliver the anti-huntingtin miRNA to neurons.
If you don’t remember what a miRNA is – or you simply don’t know, this is not a blog for experts! – click here for a brief overview.
1) SILENCING TOXIC GENES (THE CASE OF AMT-130)
PROBLEM: a mutation results in the production of a toxic protein.
SOLUTION: blocking the translation of the mRNA derived from the mutated gene (target mRNA).
HOW: designing an artificial miRNA (amiRNA) – a sequence fully complementary to the target mRNA and sandwiched by the flanking regions of an endogenous miRNA, so that the amiRNA is processed like cellular miRNAs. Delivered by a rAAV, the amiRNA anneals to the target mRNA and causes its degradation. The level of toxic protein is reduced, the mutated gene has been silenced.
NOTE: multiple amiRNAs can be combined to potentiate gene silencing. However, this solution comes with at least two dowsides:
- increases the chances of silencing unintended genes as well (off-target silencing),
- may cause a reduction in endogenous miRNAs as the the cellular pathways responsible for their processing is saturated by the artificial constructs.

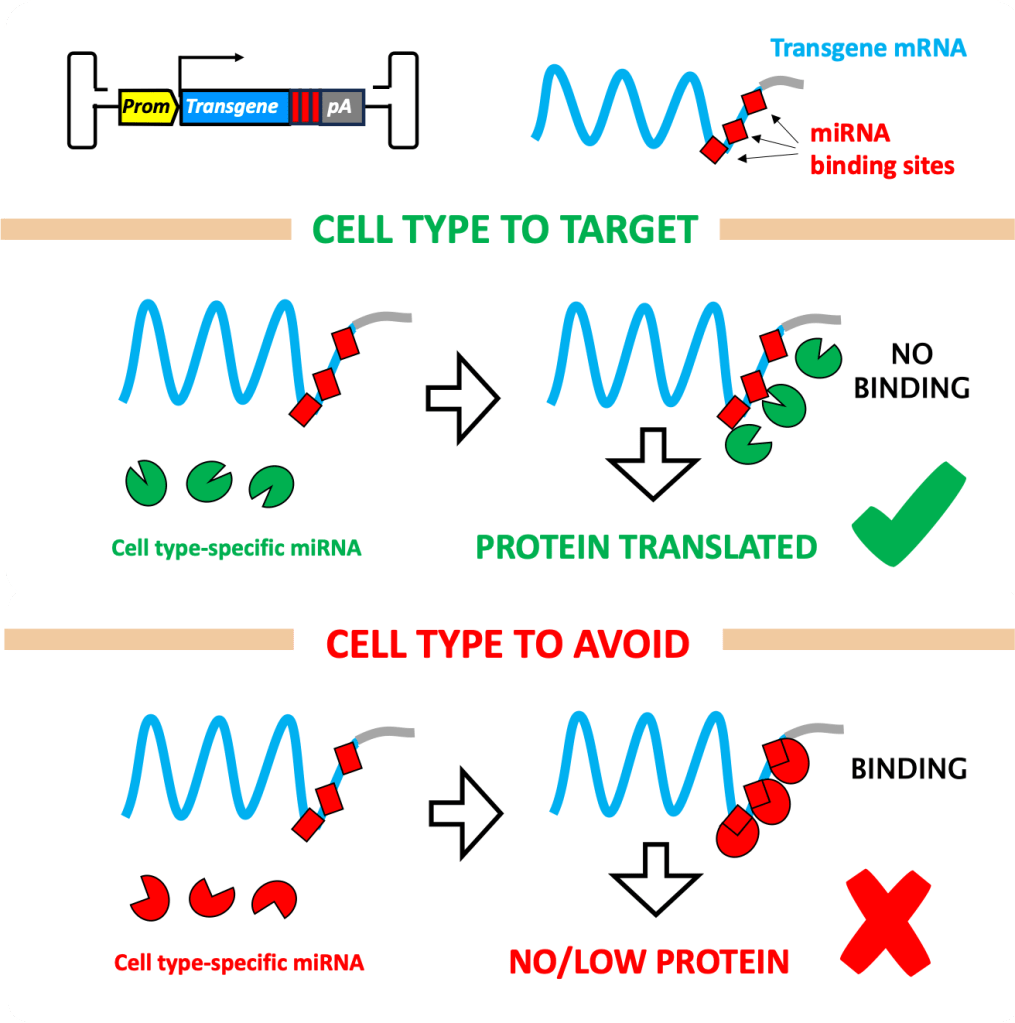
2) DETARGETING TRANSGENE EXPRESSION
PROBLEM: the transgene in the rAAV is toxic in certain cell types.
SOLUTION: building a “safety-switch” in the transgene that induces its silencing in these cells (cells to avoid).
HOW: the safety-switch is a set of sequences to which specific miRNAs bind (known as miRNA response elements, or mREs), silencing the transgene. These miRNAs are present only in the cells to avoid, so that only there the transgene is silenced. This approach enables scientist to detarget transgene expression from desired cell types and tissues.
NOTE: this is not the only strategy to restrict the expression of a transgene to a subset of cells, not the most popular. More popular approaches include and cell type-specific promoters.
- the development of rAAVs with refined tropism (its capsid is engineered so that it preferentially infects specific cell types),
- the use of cell type-specific promoters (the rAAV infects multiple cell types, but the transgene is expressed only in those with the appropriate transcription machinery).
3) SELF-REGULATING TRANSGENE
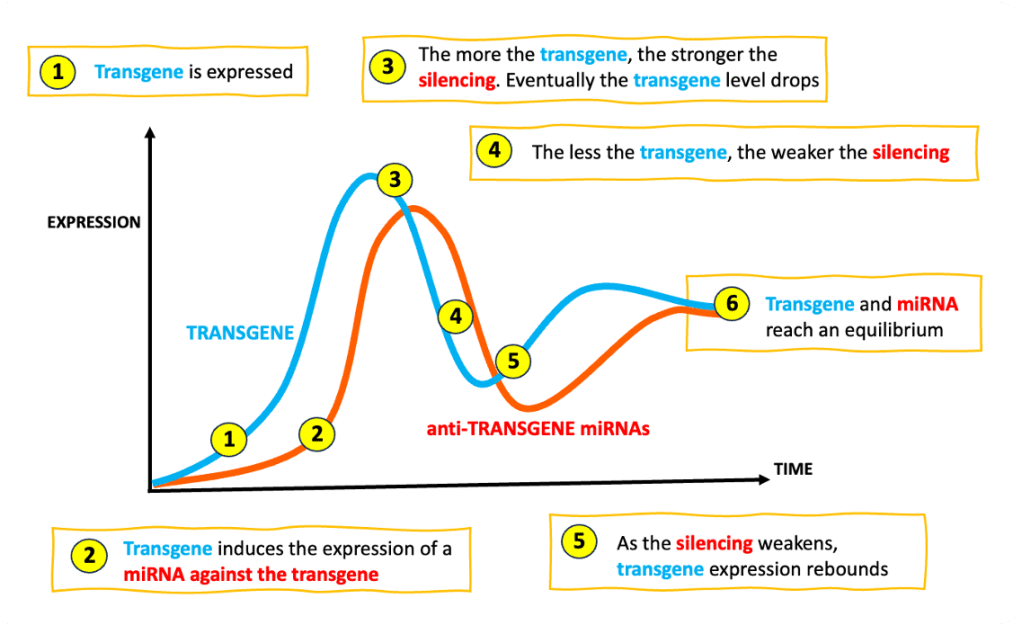
PROBLEM: the transgene is over-expressed and it becomes toxic.
SOLUTION: using a transgene that regulates its own expression.
How: identify miRNAs that cells up-regulate when expressing the transgene in the rAAV and incorporate mREs for these miRNA in the transgene tail (the 3’ UTR). Now, transgene and miRNAs form an inhibitory loop: the more the transgene is expressed, the stronger its silencing.
NOTE: this solution was pioneered in the development of a gene therapy for Rett syndrome. Researchers equipped a transgene with a panel of carefully selected mREs to prevent over-expression. This approach was named miRARE, for miR-responsive auto-regulatory element [2].
REFERENCES
- Kaiser J. In a first, a gene therapy seems to slow Huntington disease. Science (News).
- Sinnett SE et al. Engineered microRNA-based regulatory element permits safe high-dose miniMECP2 gene therapy in Rett mice. Brain. 2021 Nov 29;144(10):3005-3019.

Leave a comment